News
We issue statements on news in society and politics with a bearing on animal ethics. We also provide information on current developments in the area of agriculture and consumption.

The “invisible” animals need your help
Acknowledging the capacity for suffering as a crucial moral criterion is the core concern of Sentience. Therefore, we aim to give a voice to those animals whose well-being receives no attention in our society - the “invisible” animals.

Animal Welfare and Swiss Agricultural Policy: What Does the Population Think?
When the Swiss people are polled regarding the objectives of agricultural policy, a key concern consistently arises: animal welfare. What actions can we take in response to these findings?

Annual Report 2023
The landmark votes on the Primate Initiative and the Initiative to Abolish Factory Farming in 2022 sparked significant attention and much-needed discussions. Last year, we were able to build on this momentum and advance our vision with broad support.

Interspecies diplomacy: ever a possibility? Decoding
Despite the current Swiss political scene, the environmental emergency continues, and its impact on all beings is evermore pressing. And so, we must start now to think about what engaging diplomatically with non-human animals will look like.

One donation, double the impact ✊
Our voice for the animals is more important than ever. Until the end of December, every donation to Sentience will be doubled. For you, this means: one donation, double the impact!
A strong alliance for animals
In collaboration with organizations that supported the initiative to abolish factory farming last year, we devoted two days to exploring ways to enhance the well-being of animals in Swiss agriculture.

Sentience becomes the hundredth member of the Open Wing Alliance
Sentience is the first Swiss organization to join the alliance, which was founded in 2016. In doing so, our organization is committing to become an even stronger advocate for the interests of chickens at home and abroad in the future.

Key Learnings 2022
Both of our initiatives represented landmark achievements for Sentience. They shaped the public discourse in 2022 and contributed to the slow inculcation of animal ethics into mainstream society. Yet, there is room for improvement.

Annual Report 2022
The past year was a huge milestone in the recent history of our association. With the Primate Initiative in Basel and the national referendum on factory farming, two proposals of historical importance came to the ballot box.

SDGs without food system reform are missing the elephant in the room
The link between farmed animal welfare and the UN Sustainable Development Goals (SDGs) is often overlooked. Many people are unwilling to accept that we need to treat ‘food animals’ differently if we want to address the climate crisis effectively.
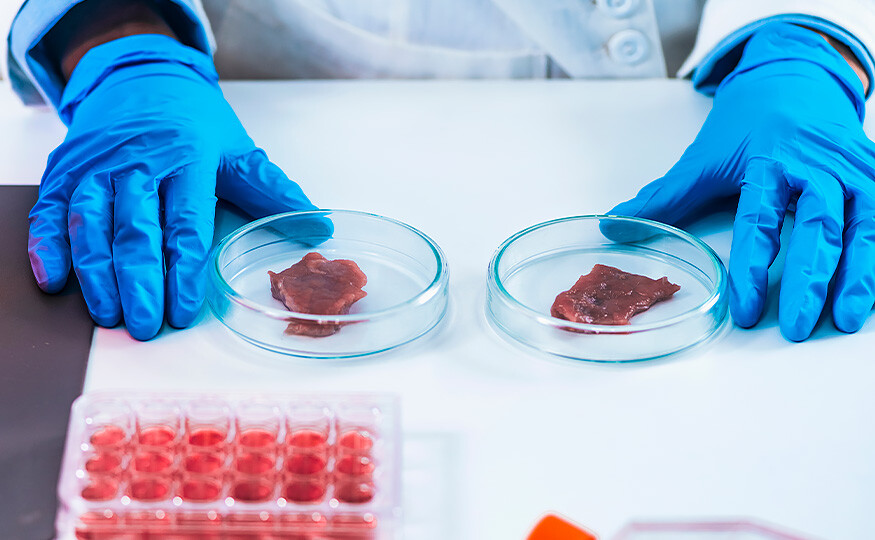
Introducing the Protein Lab 2023
We are pleased to introduce our first fully-funded project for 2023. Protein Lab comprises a series of one-day workshops where experts will come together to discuss how our food system can transition towards plant-based protein sources.

Meet the team who will take our work forward in 2023
The most eventful year so far is coming to an end for Sentience. Get to know our new team and support our Matching Challenge so that we can continue our commitment to non-human animals.

Double Your Donation by December 31
We would like to announce an exciting giving opportunity! From now until the end of the year, all donations to Sentience will be matched up to a total of 30,000 CHF. This matching challenge is made possible thanks to a few generous donors...

We lost the vote, and factory farmed animals still need our help
Swiss voters have now decided the outcome of our flagship initiative to abolish factory farming, which was launched by Sentience in 2018. The incredible support of our partner organisations, network of volunteers and public figures made it possible…

Despite defeat: Factory farming has won Switzerland’s attention
The Swiss electorate rejected the initiative against factory farming. As part of the sponsoring committee, Sentience is pleased that the initiative has received a great deal of attention in recent months. Since the initiative's launch in 2018…
